The Earth's atmosphere exerts a pressure upon us, known as the atmospheric pressure, which can be measured in a number of ways. At sea level the standard pressure is 14.7 psi or 29.92" of mercury (Hg) or 760 mm of mercury (Torr). Because the barometric pressure varies, the above sea level pressures are used as a reference point.
The term "vacuum" is used to describe the zone of pressure below atmospheric
pressure.
In the USA the common standard to measure rough vacuum is inches of
mercury ("Hg), which can be measured in two different ways. One method is as "Hg
gauge ("HgV), where the scale starts at 0"Hg (atmospheric pressure) and goes up
to 29.92"Hg, which is perfect vacuum. The other way is to measure in "Hg
absolute ("HgA), which is a gauge with a reversed scale. In this case the scale
on the gauge reads 29.92"Hg at atmospheric pressure and 0"Hg would be perfect
vacuum. Please note that a perfect vacuum is not possible on earth, no matter
which vacuum pump is used.
An absolute pressure gauge reading in Torr reads 760 Torr at atmospheric pressure, which is zero vacuum and would read 0 Torr at perfect vacuum.
Low grade vacuum may be reached using a variety of means. In the range to several 10s of Torr, sealed reciprocating piston compressors may be used. Piston compressors have the disadvantage of the dead space which exists above the piston. This, plus leakage past the piston, limits the degree of vacuum that can be achieved.
Better vacuum may be obtained with a rotary, oil sealed pump. This type
of pump has a rotating off-centre cylindrical rotor that "sweeps" air through
the cylindrical housing in which the rotor is located. Air is kept from passing
from between the vacuum and pressure sides by means of either a set of two vanes
which are arranged across the diameter of the rotor or by means of a sliding
single vane mounted in the housing.
At high vacuum, air doesn't respond very well to being squeezed and pushed around by pistons and rotors. At these pressures gas molecules don't really flow. Instead they more or less wander into the pump. The most common type of pump for use in the high vacuum realm is the diffusion pump. Diffusion pumps are simple, quiet and only require simple (but sometimes tedious) maintenance.
For Ultra High Vacuum see UHV.
![]() The unit of pressure
The unit of pressure
The SI unit of pressure is the Pascal (
1 Pa = 1 N/ m-2 )
Normal atmospheric pressure ( 1 atm.) is 101325
Pa or 1013 mbar ( 1 bar = 105 Pa ).
An older unit of pressure is the Torr (
1 Torr = 1 mmHg ). One atmosphere is ca. 760 Torr ( i.e. 1 Torr = 133.3 Pa ).
| 1 atmosphere = 1.0133 bar |
|
1 atmosphere = 760 torr |
| 1 atmosphere = 1.0133 x 105 Pa |
| 1 torr = 1 mm Hg |
| 1 micron Hg = 1 milliTorr |
| 1 millibar = 100 Pa |
| 1 torr = 133.32 Pa |
| 1 millibar = 0.75 Torr |
Classification of the degree of vacuum is hardly an exact science - it very much depends upon who you are talking to - but as a rough guideline :
| Rough (low) vacuum | 1 - 10-3 Torr |  |
| Medium vacuum | 10-3 - 10-5 Torr |  |
| High vacuum (HV) | 10-6 - 10-8 Torr | |
| Ultrahigh vacuum (UHV) | < 10-9 Torr |  |
a) The molecular density, n
The gas density is easily obtained from the ideal gas law :
n = ( N / V ) = P / (k.T) [ molecules/m-3 ]
where : P - pressure [ N/m-2 ]
k - Boltzmann's constant ( =
1.38 x 10-23 J/K )
T - temperature [ K ]
b) The mean free path
Mean Free Path. Reduction in pressure results in a lower density of gas molecules. Given a certain average velocity for each constituent molecule of air at a given temperature (at room temperature this is about 1673 km/hr) an average molecule will travel a certain distance before it interacts (collides) with another at any given pressure. This average distance between collisions is the mean free path. At 1 Torr in air this distance is about 0.005 cm, a value that scales directly with pressure.
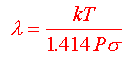 [m]
[m]
where :
P - pressure [N/m-2]
k -
Boltzmann's constant ( = 1.38 x 10-23 J/K )
T - temperature [ K
]
s - collision cross section [ m2 ]
c) The time for monolayer formation, t
The time for minolayer formation is the time required for a freshly-formed surface to become covered with a monolayer of gas molecules. An idealized value for this time t can be obtained by assuming that the surface is atomically smoth, so that the macroscopic area equals the microscopic area, and that every gas molecule that strikes it sticks to it. according to calculation equation:
| a few seconds at 10-6 torr |
| a few minutes at 10-8 torr |
| about an hour at 10-9 torr |
e)Variation of Parameters with Pressure
|
Degree of Vacuum |
Pressure (Torr) |
Gas Density (molecules m-3 ) |
Mean Free Path (m) |
Time / ML |
| Atmospheric |
760 | 2 x 1025 |
7 x 10-8 |
10-9 |
| Low |
1 |
3 x 1022 |
5 x 10-5 |
10-6 |
| Medium |
10-3 |
3 x 1019 |
5 x 10-2 |
10-3 |
| High |
10-6 | 3 x 1016 |
50 |
1 |
| UltraHigh |
10-10 |
3 x 1012 |
5 x 105 |
104 |
![]() Why vacuum in electron microscopy?
Why vacuum in electron microscopy?
There are three main reasons why the microscope column must be operated under very high vacuum:
The first of these is to avoid collisions between electrons of the beam and stray molecules. Such collisions can result in a spreading or diffusing of the beam or more seriously can result in volatization event if the molecule is organic in nature. Such volatizations can severely contaminate the microscope column especially in finely machined regions such as apertures and pole pieces. These deposited materials will serve to degrade the image.
A second reason is to avoid discharging between the cathode and the anode. There exists a very high voltage differential between these two components and stray air or gas molecules can act as carriers between the two. In conventional capacitors non-conducting oil or some other stable insulator is placed between the cathode and the anode. In an electron microscope the high vacuum serves this insulating purpose.
Finally, the area surrounding the electron emitter must be kept free of gas molecules especially oxygen. If it were not the life of the thermionic emitter would be greatly shortened and in the case of field emission we would not be able to generate electrons at all.
![]() What is Ultra High Vacuum (UHV)?
What is Ultra High Vacuum (UHV)?
UHV conditions are
generally regarded as being in the region below 10-9 millibar. Since
atmospheric pressure is about 1 bar, this means that the number of atoms of gas
in a UHV chamber is 1/1,000,000,000,000 that of air per unit volume. Frequently
pressures are a factor 10 or more below this. UHV is needed for surface science
as molecules in the air will land on a surface and change its properties. Even
at a pressure of 10-6 millibar, a layer of gas atoms will form on the
surface in about 3 seconds (assuming every atom to strike the surface sticks to
the surface). This is clearly not enough time to do an experiment. However, at
UHV pressures, which are a factor 1000 and above lower in pressure, means that
hours are needed before the sample is significantly degraded.
In order to achieve UHV, some special procedures are needed. Initially, the
vacuum chamber will be pumped down to 10-2 millibar using a rotary
pump. Then the chamber will be pumped down to about 10-6 millibar
using a Turbomolecular pump. At this stage, the vacuum chamber is baked to a
temperature of about 180 Celsius. After a day or so of baking, the ovens are
removed, and the chamber allowed to cool down again. Once down at room
temperature, the chamber should have a pressure in the UHV region. The process
of baking removes gas atoms which are stuck to the chamber walls. These gas
atoms slowly desorb from the chamber wall surfaces, and if the chamber was not
baked, then months would have to pass before the chamber achieved UHV
conditions.